LED full form is Light Emitting Diode. It is a p-n junction diode that gives off visible light or infrared (invisible) light when forward biased. The working of LED is based on Electroluminence i.e. conversion of electrical energy into light. LED light is emitted due to the large number of recombination of electron and hole. The symbol of LED is shown in figure 1.
LED Construction
Light Emitting Diode are made by combination of elements like gallium, arsenic and phosphorous. Silicon and germanium are not used to make LED because the energy released during recombination at the p-n junction is dissipated in the form of heat. The emitted light is insignificant. By using different combination of gallium, arsenic and phosphorous, it is possible to produce light of different wavelengths (or colours).
LED Material
The following table shows the different material used to make LED which gives different colour. The material can produce light having different colour depending upon the concentration of elements present. For example, GaAsP gives orange light when phosphorous concentration is less while it gives yellow light when phosphorous concentration is more. Gallium Arsenide (GaAs) produces infrared light.
| S.No. | Material | Colour |
| 1. | Aluminium Indium Gallium Phosphide (AlInGaP) | Amber, Yellow |
| 2. | Gallium Arsenic Phosphide (GaAsP) | Red,Orange, Yellow |
| 3. | Gallium Nitride (GaN) | White |
| 4. | Aluminium Gallium Phosphide (AlGaP) | Green |
| 5. | Indium gallium nitride (InGaN) | Blue, Blue-green |
| 6. | Aluminium Gallium Arsenide (AlGaAs) | Infrared |
| 7. | Zinc Selenide (ZnSe) | Blue |
LED Working
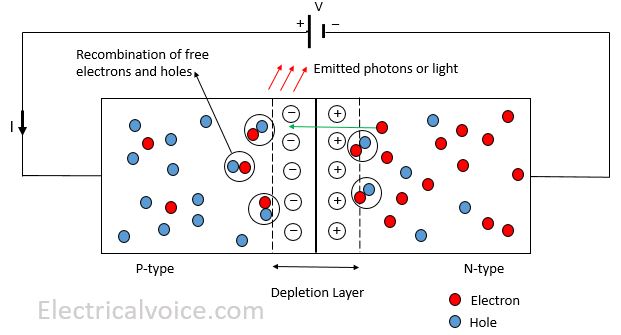
When the LED is forward biased (i.e., anode is positive w.r.t. cathode), the electrons in the n-type material are attracted by the positive terminal of the supply. Electrons cross the p-n junction and reach in the p-type material. Many of these electrons recombine with the holes near the junction. Since electrons were in the conduction band before recombination. Hence, electrons release energy in the form of light (or emission of photons) and heat while recombining with holes. Since LED is made of gallium, arsenic and phosphorous elements combination, most of the energy converted into light energy as compared to heat. The colour of the light is determined by the band gap energy of the semiconductor material.
When the LED is reverse biased (i.e., anode is negative w.r.t. cathode), LED works as a normal p-n junction diode.
Important Points
1. The operating forward voltage of LED varies from 1V to 3V.
2. The forward current ratings vary from 20mA to 100mA.
3. When forward current is increased from 0 to 20mA then efficiency of LED increases. Hence it will emit more and more light. Efficiency of LED is directly proportional to forward current.
4. When forward current is greater than 20mA then temperature increases and LED is heated up. Hence efficiency of LED decreases as temperature increases.
5. Modern LED can be made of direct band gap as well as indirect band gap semiconductor materials under controlled doping.
6. LED power dissipation is of the order of milli Watts (mW).
LED advantages
1. Light Emitting Diode response time is in microseconds.
2. It has fast ON-OFF switching.
3. LED has longer operating life (1,00,000 + hours).
4. Light Emitting Diode size is small.
5. LED is faster than LCD (Liquid crystal display).
6. It requires low voltage to operate.
7. As compared to other sources of light, LED radiate very little heat in the form of infrared radiation.
8. Light Emitting Diode can emit light of different colours without using any color filters. It depends upon the construction material.
9. LEDs emit more lumens per watt than incandescent light bulbs.
LED Disadvantages
1. LEDs are expensive.
2. The efficiency of LEDs decreases as the electric current increases.
LED Applications
1. It is used in remote controls.
2. It is used for lightening purpose.
3. It is used in electronic display.
4. It finds application in camera flashes.
