The Daniell cell is a battery which is named after the British chemist and meteorologist John Frederic Daniell, who invented it in 1836. The Daniell cell e.m.f. is 1.12 volt. This cell is used in experiments where a continuous and constant current is required.
Construction
The Daniell cell diagram is shown in figure 1. It consists of a cylindrical copper vessel containing a saturated solution of copper sulphate (CuSO4). To keep the solution saturated, it is provided with a shelf on which crystals of copper sulphate are placed. The copper vessel itself acts as a positive electrode. A porous pot containing dilute sulphuric acid (H2SO4) and an amalgamated zinc rod (impure zinc rod covered with a layer of mercury) is placed in the CuSO4 solution. Zinc rod acts as a negative electrode.
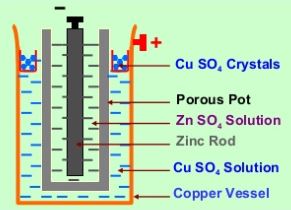
Anode (negative electrode): Zinc (Zn) rod
Cathode (positive electrode): Copper (Cu) vessel
Depolariser: Copper sulphate (CuSO4)
Daniell Cell Working
When zinc rod and copper vessel are connected to the external circuit, the following reactions take place in the cell.
(a). Dilute H2SO4 acid dissociates into ions
H2SO4 → 2H+ + SO42−
(b). some of the zinc atoms go into solution as ions
Zn → Zn2+ + 2e−
(c). Zn2+ ions and SO42− ions give ZnSO4
Zn2+ + SO42− → ZnSO4
combining equations (a), (b), and (c), we get
Zn + H2SO4 → ZnSO4 + 2H+ + 2e−
The H+ ions diffuse through the porous pot and react with CuSO4 to give H2SO4 and Cu2+ ions are liberated i.e.
2H+ + CuSO4 → H2SO4 + Cu2+
These Cu2+ ions are deposited on the copper vessel making it positive with respect to the zinc electrode. Since CuSO4 is reacting with hydrogen ions (H+) to give liquid (i.e. H2SO4) so there is no polarization effect. Hence CuSO4 solution acts as a depolariser.
The e.m.f. of a Daniell cell is 1.12 Volt and its internal resistance is very low.
Frequently Asked Questions
-
Why we use Daniell cell in an experiment in which continuous and constant supply of current is required?
We use Daniell cell in an experiment in which continuous and constant supply of current is required because the concentration of CuSO4 remains the same, so the cell supplies continuous and constant current as e.m.f. of the cell remains constant.
-
What do you mean by amalgamated zinc rod?
The impure zinc rod covered with a layer of mercury is called amalgamated zinc rod.
-
Name the depolariser used in the Daniell cell.
The depolariser used in the Daniell cell is copper sulphate (CuSO4).
