The Leclanche cell is a battery which is named after the French scientist Georges Leclanché who invented it in 1866. The Leclanche cell e.m.f. is 1.5 volt. The application of the Leclanche cell was in electric bells, signalling, and telegraphy.
Construction
Leclanche Cell consists of a glass vessel containing a saturated solution of ammonium chloride (NH4Cl) as shown in figure 1. An amalgamated zinc rod (impure zinc rod covered with a layer of mercury) and a porous pot containing a carbon rod packed in a mixture of manganese dioxide (MnO2) and powered coke are placed in the solution of NH4Cl.
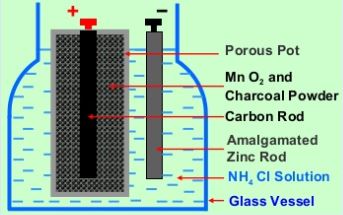
The porous pot is sealed at the top with pitch. Zinc rod forms the negative electrode and the carbon rod forms the positive electrode of the cell. Here MnO2 acts as a depolariser and powered coke makes MnO2 electrically conducting.
Anode (negative electrode): Zinc (Zn) rod
Cathode (positive electrode): Carbon (C) rod
Depolariser: Manganese dioxide (MnO2)
Electrolyte: Ammonium chloride (NH4Cl)
Leclanche Cell Working
When zinc and carbon rods are connected to the external load, the following reactions take place in the cell.
(a). Zn → Zn++ + 2e−
(b). 2NH4Cl → 2NH3 ↑ + 2H+ + 2Cl−
(c). Zn++ + 2Cl− → ZnCl2
combining equations (a), (b), and (c), we get
Zn + 2NH4Cl → ZnCl2 + 2NH3 ↑ + 2H+ + 2e−
The negative charge (2e−) released is collected by the zinc rod and it becomes negatively charged. Ammonia gas (2NH3 ↑) escapes.
The hydrogen ions (2H+) diffuse through the porous pot and react with MnO2 as follows
2H+ + 2MnO2 → Mn2O3 + H2O + 2e+
The positive charge (2e+) is acquired by the carbon rod which becomes positively charged. Mn2O3 formed in the porous pot is again converted into MnO2 by absorbing oxygen (O2) from the air when left to itself for some time.
The potential difference between the negatively charged zinc rod (anode) and positively charged carbon rod (cathode) is set up. The e.m.f. of Leclanche cell is 1.5 volt.
Limitations
1. The internal resistance of this cell is high.
2. This cell can not supply continuous current for a long time but is used only for intermittent works.
Frequently Asked Questions
Q1. Why can’t we use Leclanche cell in an experiment in which continuous and constant supply of current is required?
Answer. we can’t use Leclanche cell in an experiment in which continuous and constant supply of current is required because its e.m.f. decreases with the passage of time and hence cannot supply constant current for long time.
Q2. What do you mean by amalgamated zinc rod?
Answer. The impure zinc rod covered with a layer of mercury is called amalgamated zinc rod.
Q3. Name the electrolyte used in the Leclanche cell.
Answer. The electrolyte used in the Leclanche cell is Ammonium chloride (NH4Cl).
Q4. Name the depolariser used in the Leclanche cell.
Answer. The depolariser used in the Leclanche cell is Manganese dioxide (MnO2).
