Radioactivity Definition
The phenomenon of spontaneous emission of radiations by heavy elements is called radioactivity. The elements which show this phenomenon is called radioactive elements.
Radioactivity was discovered accidentally by Antoine Henri Becquerel in 1896.
α-particle: It is called as alpha particle. It is a helium nucleus having two protons and two neutrons ( ![]() ).
).
β-particle: It is called as beta particle. It is a fast moving electron (![]() ).
).
Note: β-particle and anti-neutrino do not exist inside the nucleus but they are created at the time of emission.
γ-rays: It is called as gamma rays. γ-rays are the packets of electromagnetic radiation energy and are known as photons. They do not have any charge and their rest mass is zero.
Properties of α-particle
1. They have positive charge equal to + 2e, where e = 1.6 × 10-19 Coulombs.
2. They are emitted with velocities ranging between 1.4 × 107 m/s to 2.2 × 107 m/s.
3. They are deflected by electric and magnetic fields.
4. They cause fluorescence in certain materials like zinc sulphide, etc.
5. They have low penetrating power.
6. The have rest mass equal to 4 times the mass of a proton.
7. They have high ionising power.
8. α-particles are capable of producing heating effect when fall on a substance and they can cause skin burns.
Properties of β-particle
1. β-particle has a negative charge equal to the charge on an electron.
2. The rest mass of a β-particle is equal to the mass of an electron.
3. They are emitted with a velocity of the order of 2.97 × 108 m/s.
4. They are deflected by electric and magnetic fields.
5. They cause fluorescence in zinc sulphide and other fluorescent materials.
6. The ionising power of β-particles is about 1/100 times the ionising power of α-particles.
7. The penetration power of β-particle is more than that of α-particle.
Properties of γ-rays
1. They are the packets of energy of electromagnetic radiations.
2. They have no charge.
3. The rest mass of γ-rays is zero.
4. They always travel with the speed of light in vacuum (3 × 108 m/s).
5. They are not deflected by electric and magnetic fields.
6. They cause ionisation but their ionising power is about 1/100 times the ionising power of β-particles.
7. They have very high penetration power. Their penetration power is 100 times greater than that of β-particles.
8. They cause less fluorescence (in substances like willimite).
Types of Radioactive Decay
There are three types of radioactive decay namely, alpha, beta, and gamma decay.
Whenever a nucleus goes through alpha decay, it transforms into a different nucleus by emitting an alpha particle in alpha decay.
Beta decay is when a nucleus decays spontaneously by emitting an electron or a positron.
We know that atoms have energy levels. Similarly, a nucleus has energy levels too. When a nucleus is in an excited state, it can transition to a lower energy state by emitting an electromagnetic radiation. Further, the difference between the energy states in a nucleus is in MeV. Hence, the photons emitted by the nuclei have MeV energies and called Gamma rays. Hence the phenomena is known as gamma decay.
Laws of Radioactive Decay (i.e. disintegration)
1. Radioactive Decay is a spontaneous process and is not affected by the external conditions such as pressure, temperature,etc.
2. When a radioactive element decays by emitting an α-particle, its position goes down by two places in the periodic table.
![]()
example:
![]()
3. When a radioactive element decays by emitting a β-particle, its position is raised by one place in the periodic table.
![]()
example:
![]()
4. When a radioactive element decays by emitting a γ-rays, its position remains the same in the periodic table.
![]()
5. The rate of disintegration of a radioactive substance is directly proportional to the number of atoms remained undecayed in the substance. This law is called radioactive decay law or disintegration law.
Consider a radioactive substance having No number of atoms before decay (i.e. at t = 0). When radioactive disintegration begins, the number of atoms in the substance decreases. Let after time t, the number of atoms remained undecayed in the substance be N. If dN is the number of atoms decayed in small time dt, then the rate of disintegration = dN/dt.
According to decay law,
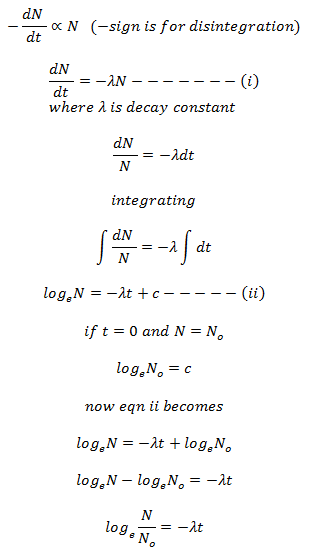
This equation is known as exponential decay equation.
Radioactive decay constant or disintegration constant (λ)
Radioactive decay constant (λ) is the reciprocal of the time during which the number of atoms in the radioactive substance reduces to 36.8% of the original number of atoms in it.
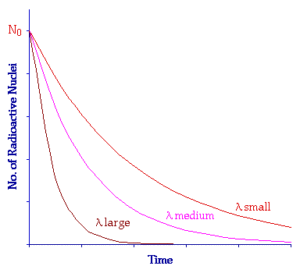
Significance of decay constant
If the value of λ of a radioactive substance is small, it will decay slowly. If the value of λ of a radioactive substance is large, it will decay rapidly as shown in figure below.
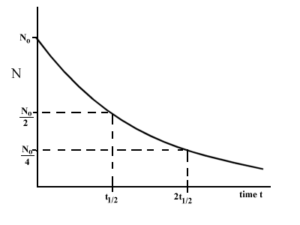
Half Life of a radioactive substance (T1/2)
The time during which half of the atoms of the radioactive substance disintegrates is called half life of a radioactive substance.
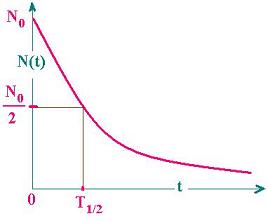
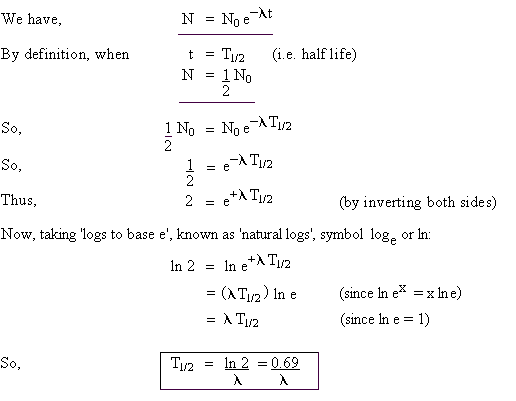
Half-life of radioactive substance is inversely proportional to the decay constant (λ).
Mean-life or Average life of a radioactive substance
The sum of lives of all atoms divided by the total number of atoms is called as mean-life or average life of a radioactive substance.
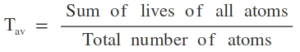
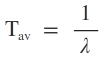
Activity of a Radioactive substance
The activity of a radioactive substance is defined as the rate of disintegration of the substance. Activity is inversely proportional to half-life.
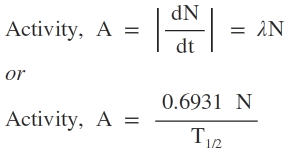
Units of activity
- Curie (Ci)
- Rutherford (rd)
- Becquerel (Bq)
1 Curie (Ci) = 3.7 × 1010 disintegration per second
1 Rutherford (rd)= 106 disintegration per second
1 becquerel (Bq) = 1 disintegration per second
Exercise
Q. Who discovered natural radioactivity?
Answer. Natural Radioactivity was discovered by Antoine Henri Becquerel in 1896.
Q. What is radioactivity?
Answer. The phenomenon of spontaneous emission of radiation by heavy elements is called radioactivity.
Q. Who discovered artificial radioactivity?
Answer. Artificial Radioactivity was discovered by Irene Curie Joliot and her husband Frederick Joliot.
Q. What is artificial or induced radioactivity?
Answer. The process by which stable nuclei are made unstable by bombarding them with high energy particles and then these unstable nuclei are made to emit nuclear radiations is called artificial or induced radioactivity.
Q. What are radioactive elements?
Answer. The elements whose atoms disintegrate and emit radiations are called radioactive elements.
Q. Name the radiations emitted by the radioactive substance?
Answer. The radiations emitted by the radioactive substance are α, β, and γ-rays.
Q. Which one has more ionising power: α-particle or β-particle?
Answer. Since velocity of α-particle is less than that of β-particle, so α-particle has more ionising power than β-particle.
Q. Can a single nucleus emit α-particle, β-particle, and γ-rays together?
Answer. No. A nucleus either emits an α-particle or β-particle and if left in the excited state, it may emit γ-ray.
Q. What is the effect of temperature and pressure on the radioactivity?
Answer. There is no effect of temperature and pressure on the radioactivity.
Q. The decay constants of two radioactive substances A and B are 4 per year and 5 per year respectively. Which of the two substances will decay fast?
Answer. As we know that decay constant, λ = (1/t)
Since decay constant λ of substance B is more than that of substance A, so substance B will take less time to decay than A. Hence substance B will decay faster than A.
Q. All radioactive substances seem to be identical. Explain
Answer. Every radioactive sample require infinite time to disintegrate completely and in this sense, all the radioactive substances are identical.
