Nickel Metal Hydride (NiMH) Battery
Contents
show
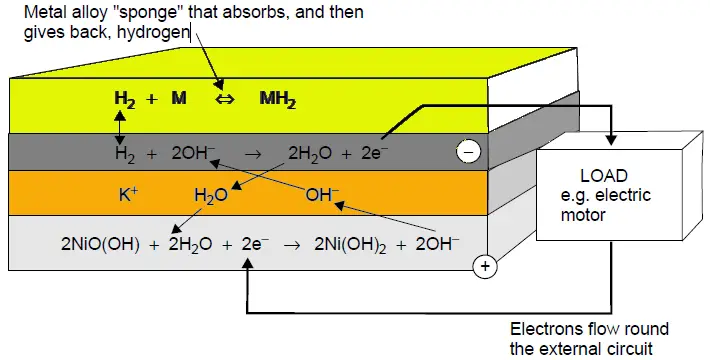
The principles in which NiMH cells operate are based on their ability to absorb, release, and transport (move) hydrogen between the electrodes within the cell.
Negative Electrode: Metal Hydride such as AB2 (A=titanium and/or Vanadium, B= Zirconium or Nickel, modified with chromium, Cobalt, iron and/or manganese) or AB5 (A = rare earth mixture of lanthanum, cerium, neodymium, praseodymium, B = Nickel, Cobalt, manganese, and/ or aluminium).
Positive Electrode: Nickel Oxyhydroxide (NiO(OH))
Electrolyte: Potassium Hydroxide (KOH)
Reactions at Electrodes
Features
- Higher energy density (40%) than NiCd
- Non-toxic
- Reduced life, discharge rate (0.2-0.5C)
- More expensive (20%) than NiCd.
Advantages
- 30%+ higher capacity compared to standard NiCd battery.
- Less prone to memory than NiCd — fewer exercise cycles are required.
- Simple storage and transportation; not subject to regulatory control.
- Nickel content makes recycling profitable.
- Non toxic. Hence, Environmentally friendly.
- Wide temperature range.
Limitations
- More complex charge algorithm needed — the NiMH generates more heat during charge and requires a longer charge time than the NiCd.
- High self-discharge — typically 50% higher than NiCd. New chemical additives improve self-discharge but at the expense of lower.
- Coulombic efficiency only about 65% (99% with Li-ion)
- Performance degrades if stored at elevated temperatures.
- Requires complex charge algorithm. Sensitive to overcharge.
- Does not absorb overcharge well; trickle charge must be kept low.
