Lead Acid Battery
A lead acid battery is an electrochemical device that produces voltage and delivers electrical current.
Negative Plate → Spongy lead (active material)
Positive Plate → Lead dioxide (active material)
Electrolyte → Dilute Sulphuric Acid
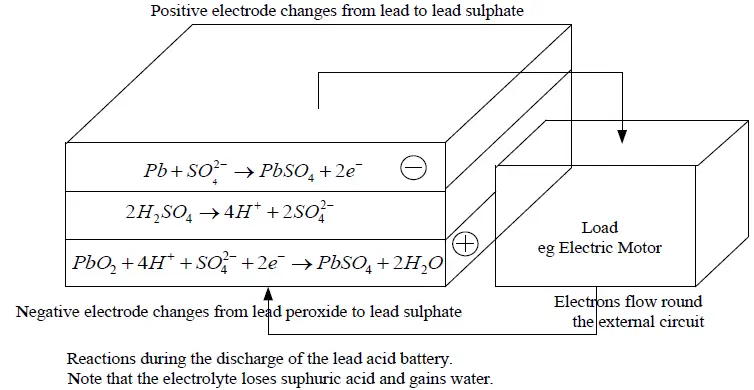
During Discharging
The sulphuric acid (H2SO4) combines with the lead and the lead dioxide to produce lead sulphate (PbSO4) and water, electrical energy being released during the process.
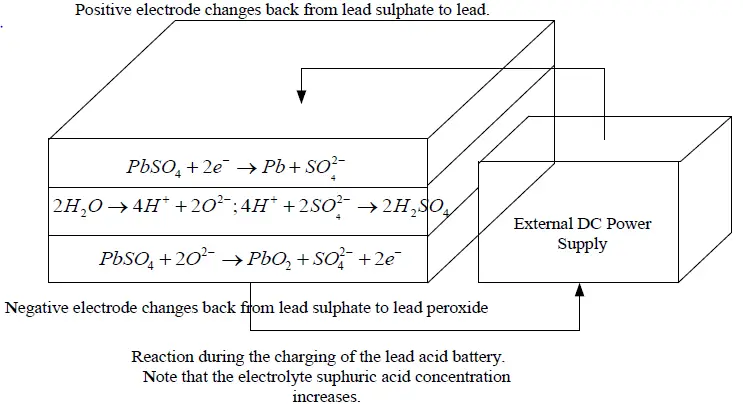
During Charging
When being charged, the electrodes revert to lead and lead dioxide. The electrolyte also recovers its sulphuric acid, and the concentration rises.
The overall reaction is:
Pb + PbO2 + 2H2SO4 ↔ 2PbSO4 + 2H2O
Reactions at Electrodes While Battery is Discharging
Reactions at Electrodes While Battery is Charging
Puekert’s law
C = Ikt
where
C = Battery Capacity at a one-ampere discharge rate
I = actual discharge current (i.e. current drawn from a load)
k = Peukert constant, determined by the battery manufacturers specification datasheet discharge curves. It is typically between 1.1 and 1.3.
t = actual time to discharge the battery in hours.
