A fuel cell is an electrochemical energy conversion device. It converts chemical energy into electrical energy, water, and heat through electrochemical reactions. The voltage generated by a single fuel cell is small (< 1 volt). So many cells are connected in series to generate the desired voltage.
Contents
show
A fuel cell has two electrodes, one is negative (called anode) and positive (called cathode). The reaction that generates electricity takes place at the electrode.
Parts of Fuel Cell
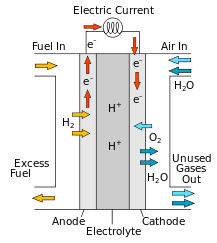
- Anode
- Cathode
- Catalyst
- Proton Exchange Membrane
Working of Fuel Cell
- Fuel (H2) is first transported to the anode of the cell.
- Fuel undergoes the anode reaction.
- Anode reaction splits the fuel into H+ (a proton) and e−.
- Protons pass through the electrolyte to the cathode.
- Electrons can not pass through the electrolyte and must travel through an external circuit which creates a usable electric current.
- Protons and electrons reach the cathode and undergo the cathode reaction.
- The oxygen reacts with H+ (a proton) and e− to form water vapour.
Reactions at Electrodes
Anode Reaction
2H2 → 4H+ + 4e−
Cathode Reaction
O2 + 4H+ + 4e− → 2H2O
Overall Cell Reaction
2H2 + O2 → 2H2O
Types of Fuel Cell
There are different types of fuel cells, differentiated by the type of electrolyte separating the hydrogen from the oxygen. The types of fuel cells are
- Alkaline fuel cells (AFC)
- Proton Exchange Membrane Fuel Cells (PEMFC)
- Phosphoric Acid Fuel Cell (PAFC)
- Solid oxide fuel cells (SOFC)
- Molten Carbonate fuel cells (MFFC)
- Direct methanol fuel cells (DMFC)
1. Alkaline fuel cells (AFC)
- It operates on compressed hydrogen and oxygen.
- Its efficiency is about 70 %.
- Operating temperature is 50 to 100°C.
- It was used in Apollo spacecraft to provide both electricity and drinking water.
- AFC require pure hydrogen fuel and have platinum electrode catalysts.
2. Proton exchange membrane fuel cell (PEMFC)
- Work with a polymer electrolyte in the form of a thin, permeable sheet.
- Efficiency is about 40 to 50 %.
- Suitable for homes and cars.
3. Phosphoric acid fuel cell (PAFC)
- Uses phosphoric acid as the electrolyte.
- Efficiency ranges from 40 to 80 %.
- Typically used for stationary power generation.
4. Solid oxide fuel cells (SOFC)
- Uses a hard, ceramic compound of metal oxides as an electrolyte.
- Efficiency is about 60%.
- Operating temperatures are about 1,000 °C, so no reformer is required for extracting hydrogen from fuel
- Utility applications
5. Molten carbonate fuel cell (MCFC)
- Uses high-temperature compounds of salt carbonates as an electrolyte.
- Efficiency ranges from 60 to 80 %.
- Operating temperature is about 650 °C.
- Developed for natural gas and coal-based power plants to generate power for industry and military use.
6. Direct Methanol Fuel Cells (DMFC)
- Use to power cellular phones and laptops.
- Use a polymer membrane as the electrolyte.
- The anode catalyst itself draws the hydrogen from the liquid methanol (no reformer needed).
- The efficiency of about 40%.
- Typically operate at a temperature between 60-90 °C.
- Higher efficiencies are achieved at higher temperatures.
Comparison between different Fuel Cell
| Fuel cell type | Mobile ion | Operating Temperature | Applications |
| Alkaline (AFC) | OH− | 50–200°C | Used in space vehicles, e.g. Apollo, Shuttle. |
| Proton exchange membrane (PEMFC) | H+ | 30-100°C | Vehicles and mobile applications, and for lower power CHP systems |
| Direct methanol(DMFC) | H+ | 20-90°C | Suitable for portable electronic systems of low power, running for long times |
| Phosphoric acid (PAFC) | H+ | 220°C | Large numbers of 200kW CHP systems in use |
| Molten carbonate (MCFC) | CO32- | 650°C | Suitable for medium to large scale CHP systems, up to MW capacity |
| Solid oxide (SOFC) | O2- | 500-1000°C | Suitable for all sizes of CHP systems, 2 kW to multi MW |
Advantages of Fuel Cell
- Physical Security
- High Reliability
- Higher Efficiency
- Hydrogen fuel cells don’t produce air pollutants or greenhouse gases
- Fuel cells can readily be combined with other energy technologies, such as batteries, wind turbines, solar panels, and super-capacitors.
- can be refueled in 5 minutes.
- 40% higher fuel economy than diesel vehicles.
Applications of Fuel Cell
- Power sources for vehicles such as cars, trucks, buses and even boats and submarines.
- Power sources for spacecraft, remote weather stations, and military technology.
- Batteries for electronics such as laptops and smartphones.
- Providing power for base stations or cell sites.
- Food preservation.
- Small heating appliances.
- Used in Hybrid vehicles
