Electric charge is a fundamental property of matter due to which electrical and other related effects are produced in the matter. The transfer of electrons from one object to another is responsible for the electric charging of the objects.
Or
Electric charge is the physical property of matter that causes it to experience a force when placed in an electromagnetic field.
1. The branch of physics which deals with electric charges at rest is called electrostatics or static electricity.
2. Charge is a scalar quantity.
3. The S.I. unit of charge is Coulomb. It is denoted as C.
4. The dimensional formula of charge is [AT].
Types of Charges
There are two types of charges i.e. positive charge and negative charge. Consider an experiment where a glass rod is rubbed with silk cloth and rubber rod is rubbed with fur.
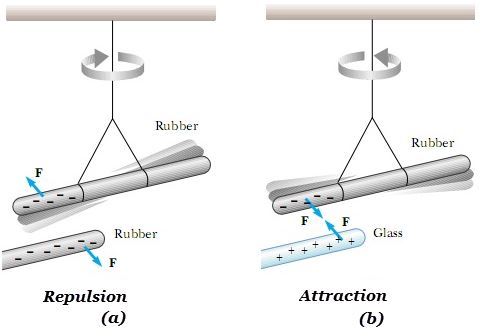
Let one end of a rubber be rubbed with fur and then the same be suspended. When another similarly charged rubber rod is brought near it, the suspended rod turns away showing repulsion as shown in fig 1(a). If the charged fur is now brought near it, the suspended rod is attracted towards it.
If a glass rod rubbed with silk is brought near the rubber rod rubbed with fur, there is attraction between the two rods as shown in fig 1(b). When silk is brought near the suspended rubber rod, there is repulsion.
The glass rod as well as fur attract the electrified rubber rod. They have the same kind of electrification. They are said to be positively charged. On the other hand, rubber and silk are said to be negatively charged.
The charge on a glass rod rubbed with silk as positive charge and the charge on rubber rod rubbed with fur as negative charge. The following points can be concluded from the experiment.
1. There is existence of two types of charges i.e. positive and negative charge.
2. Like charges repel each other.
3. Unlike charges attract each other.
Note: Positive charges are denoted by a (+) sign and negative charges by a (−) sign. The property which differentiates the two kinds of charges is called the polarity of charge.
It is noted that equal amounts of two kinds of charges cancel the action of each other and produce an electrically neutral body.
Methods of Charging
Every neutral body consists of positive and negative charges. These charges are normally equal and opposite in a body so net charge in the body is zero. Making a body to acquire property of attracting small objects is called charging(or electrification). A body can by charged by the following ways:
1. Charging by physical contact or charging by conduction
2. Charging by friction or rubbing
3. Charging by induction
Charging by friction or rubbing
When a body is rubbed with another body, both of them charged. One of the bodies acquires positive charge and the other acquires negative charge. For example, when a glass rod is rubbed with a silk, the glass rod acquires positive charges,and at the same time, the silk,the silk acquires negative charges.
Charging by physical contact or charging by conduction
When an uncharged body is made in contact with a charged body flow into the non charged body and the body is charged. For example ,if an uncharged sphere A is made in contact with a charged sphere B, the sphere A will be charged sphere B, the sphere A will be charged with the same charge as in the sphere B.
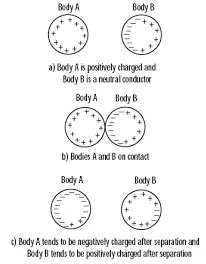
Charging by induction
When a charged particles or body is brought near non charged body without touching, charges are developed in the uncharged body. This method of charging a body is called charging by induction.
Note: 1. An infinitesimally small positive charge experiencing electric force is called a test charge. A unit positive charge is commonly used as a test charge.
2. If the sizes of charged bodies are very small as compared to the distances between them, we treat them as point charges.
Basic Properties of Charge
1. Quantization of Electric charge
Quantization is the property of an electric charge which tells that any charged body can have charge which is an integral multiple of the basic charge ‘e’.
q = ±ne
q= total charge
n = 1,2,3,4….
e = charge on electron i.e. 1.6 × 10–19 C
Charge cannot exist like ±e/2,±e/3,….
Millikan’s oil-drop experiment proves the existence of charges as integral multiple of basic charge e.
2. Additivity of Electric charge
Electric charge is additive. Total charge on an extended body is the algebraic sum of charges in different regions of the body.
3. Invariance of Electric Charge
Electric charge is independent of frame of reference i.e. charge on a body does not vary whatever may be its speed or the speed of the observer i.e. Charge at rest = Charge at motion
4. Conservation of Electric Charge
Charge can neither be created nor destroyed in an isolated system.
Difference between Electric Charge and Mass
| S.No. | Electric Charge | Mass |
| 1. | Electric charge may be positive or negative quantity. | Mass of a body is always a positive. |
| 2. | Charges can be added like real numbers so they can be treated as scalars. | Masses can also be added like real numbers and are scalars. |
| 3. | Charge is quantized. | Mass of a body is not considered to be quantized. |
| 4. | Charge is always conserved. | Mass is not conserved as mass can be changed into energy and vice-versa according to the relation
E = mc2 |
| 5. | Charge on a body is not affected by the velocity of the body i.e. charge is invariant. q(at rest) = q(in motion) |
Mass of a body changes with velocity of the body. |
| 6. | Force between charges may be attractive (in case of unlike charges) or repulsive (in case of like charges). |
Force between masses is always attractive. |
| 7. | It plays an important role in electricity. | It has an important role in gravitation. |
| 8. | In SI, charge is a derived physical quantity. | In S.I., mass is a fundamental physical quantity. |
| 9. | Charge may not exist without mass. | Mass exists without net charge also. |
